The third energy level has 3s x1. The p sublevels are named 2p 3p and 4p since the p sublevel appears only starting the 2nd level.

Solved 3 What Is The Primary Difference Between A 2p And A Chegg Com
Predict how many nodes and how many nodal planes a 4p orbital will have.

. Nodes can be either angular or radial. In the case of hydrogen the orbital which is called 1s is the one which is occupied by the hydrogen electron. Thus s p d f.
Explain how 4p orbitals are different from 3p orbitals. Advertisement Answer 0 06gavinlim. There are similar orbitals at subsequent levels - 3p x 3p y 3p z 4p x 4p y 4p z and so on.
You have probably noticed that the total number of nodes in an orbital is equal to n 1 where n is the principal quantum number. Also the 2s and 3p orbitals would. The order of the increase in energy along the various orbitals is stated as 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f.
A spin of an electron B orbital shape C principal energy level D speed of an electron 2 If the spin of one electron in an orbital is clockwise what is the spin of the other electron in that. This is because of the energy present on the level. For all those orbitals that belong to the same subshell it is the same and those orbitals that are with the same energy are stated as degenerate orbitals.
The 2p orbitals have more energy than the 2s orbital. As in s orbitals the energy and size of p orbitals increases with an increase in the quantum number ie. Thus the s sublevel which has only one orbital can have only two electrons.
How would the 2s and 3p orbitals differ from the 1s and 2p orbitals. 1 The letter p in the symbol 4p3 indicates the ___. Factors affecting the Orbital Energy.
Here 1represents the first. The next energy level the second energy level has four orbitals. An orbital can contain a maximum of two electrons.
The energy of the orbital is independent of the azimuthal quantum number. 4p orbitals are larger in size than 3p orbitals and contain additional nodes. The P shell can have 3 orbitals each holding 2 electrons and are orientated perpendicular to each other along each axis as seen below.
It is half-filledTwo such 1s orbitals from the two hydrogen atoms having electrons with opposite spins approach each other then the potential energy of the system. Similar patterns are followed in other sublevels as well. 2s and three p orbitals.
The orbitals p d and f have separate sub-levels which will therefore. In the hydrogen atom all the orbitals within a shell have the same energy. Each of the p sublevel has 3 orbitals allowing them to contain 6 electrons as each orbital may hold two.
The 1s orbital is a sphere and the 2p orbital is made up of three dumbbells oriented in the x y and z direction. Hydrogen 1s 1 atom has 1s orbital containing a single electron ie. 4p orbitals are larger in size than 3p orbitals and contain no nodes.
The 2s and 3p orbitals would have more nodes than 1s and 2p orbitalsWhat are two differences between a 2p and a 3p orbitalThe 3p orbitals have the same general shape. Thus all orbitals with designation 3 have 3 zones where the probability of finding an electron is zero. 3s has 2 spherical nodes the 3rd zone is infinite distance from the atom 3p has one planar and 1 spherical node.
By the Aufbau principle 3p will be filled first before 4p. There are four different orbitals in chemistry s p d and f that have different sizes and one orbital will accommodate up to two electrons. Such orbitals which have the same energy are called degenerate orbitals.
Will the three electrons in the 3p atomic orbitals possess the same or different values of the spin quantum number. 3d has 2 planar nodes giving it the familiar 4-lobed shape. The only electrostatic attraction between the electron and proton exists.
O 3p orbitals are larger in size than 4p orbitals and contain additional nodes. S orbital is spherical in shape and overlapping takes place to some extent in all directions. 4p 3p 2p.
4p orbitals are larger in size than 3p orbitals and contain less nodes. Thus a 2p orbital has 1 node and a 3p orbital has 2 nodes. Hence s -s bond is non directional.
All levels except for the first level have p orbitals. The 2s orbital would be the same shape as the 1s orbital but would be larger in size and the 3p orbital would have the same shape as the 2p orbitals bout would be larger in size. The 3p orbitals have the same general shape and are larger than 2p orbitals but they differ in the number of nodes.
At the higher levels the lobes get more elongated with the most likely place to find the electron more distant from the nucleus. This means in total the 4p orbital can hold 6 electrons all of which are located in the 4th shell. Using the concepts of shielding and penetration explain why a ground state configuration of 1s22s1 for an Li atom is energetically preferred over 1s22p1.

Solved Question 18 How Is A 4p Orbital Different From A 2p Chegg Com

Notes On Atomic Orbitals Shapes And Energies Cbse Class 11 Chemistry
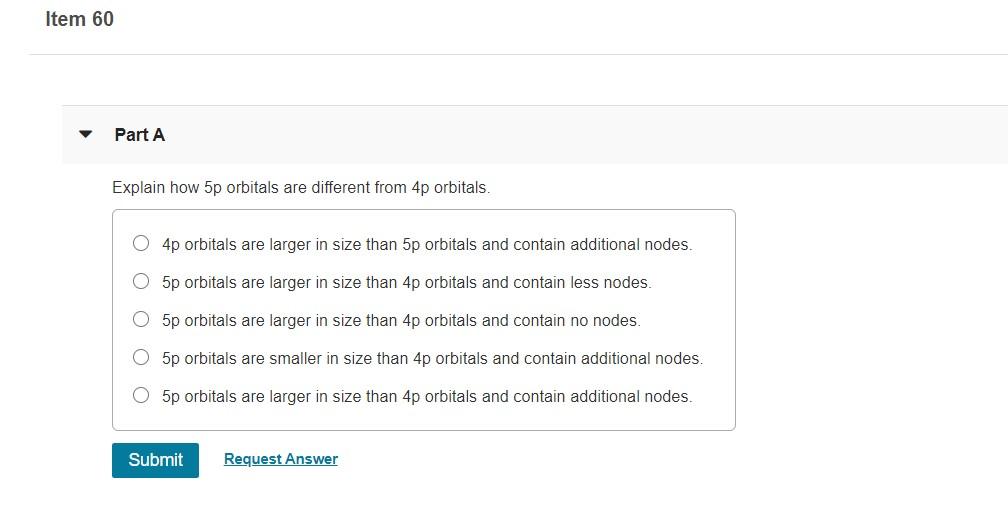
Solved Item 60 Part A Explain How 5p Orbitals Are Different Chegg Com

1 Rjc Orbital Theory The Space Around A Nucleus In Which An Electron Is Most Likely To Be Found Is Called An Orbital The Space Around A Nucleus In Which Ppt Download
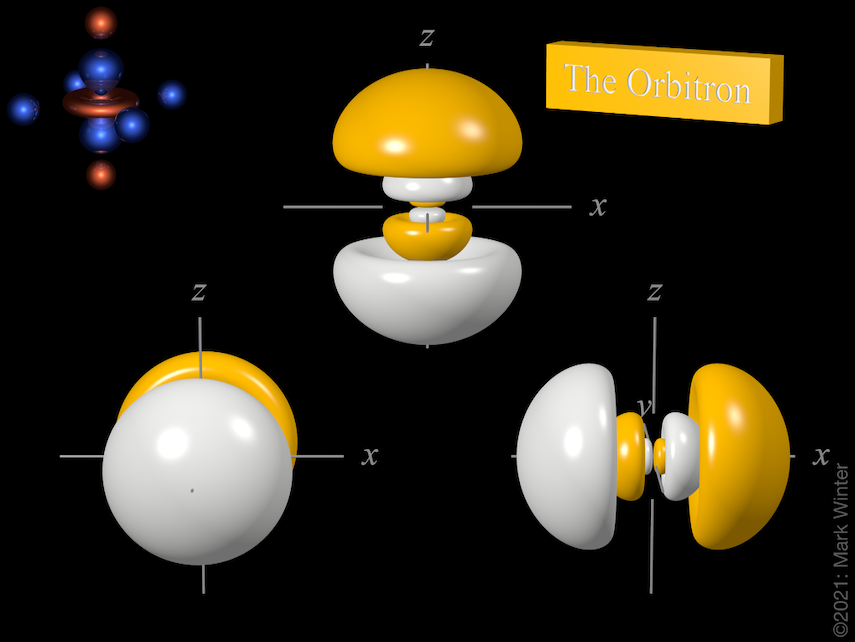
The Orbitron 4p Atomic Orbitals

Solved And 1 What Are The Shapes Of S P And D Orbitals Chegg Com
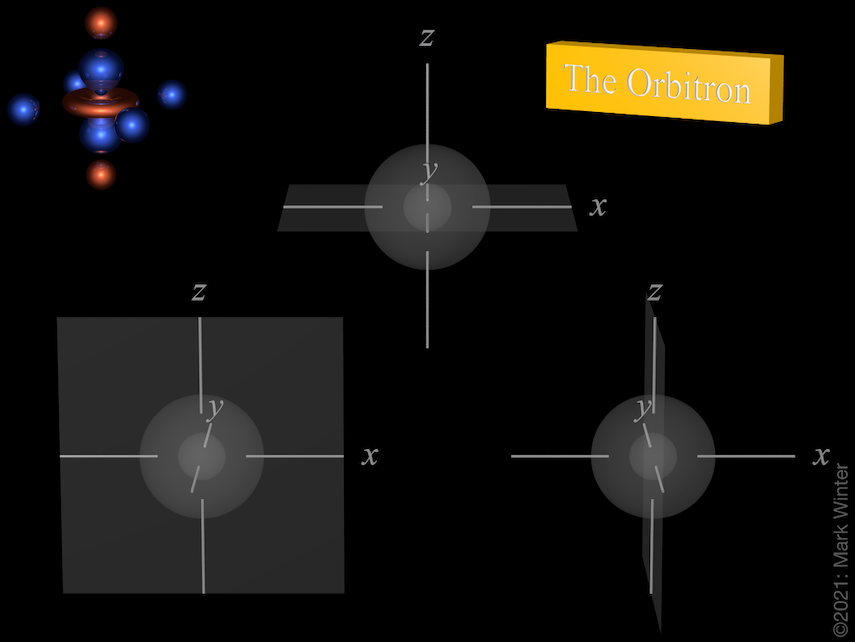
The Orbitron 4p Atomic Orbitals
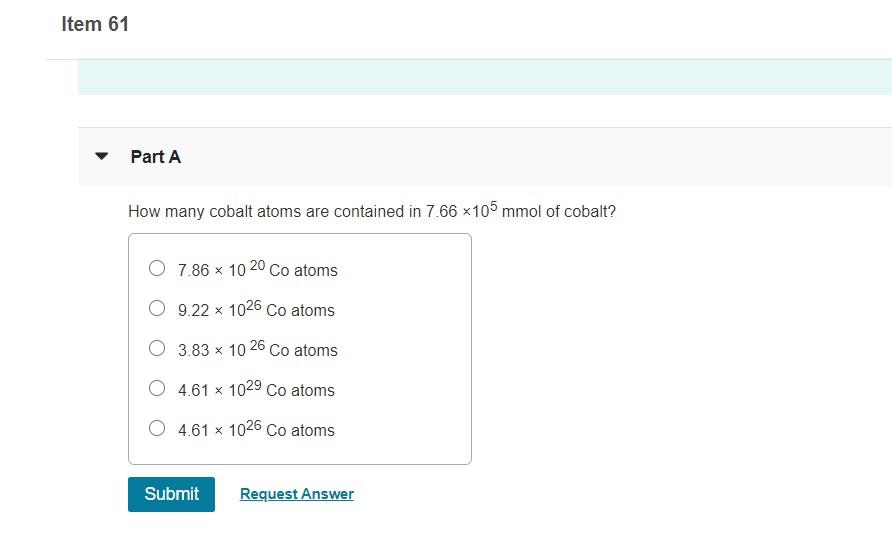
Solved Item 60 Part A Explain How 5p Orbitals Are Different Chegg Com

Solved Explain How 4p Orbitals Are Different From 3p Chegg Com
0 Comments